Gas Electron Diffraction and Small Molecule Structure
Prof. Norbert W. Mitzel, Universität Bielefeld
Gas-phase electron-diffraction is one of the most powerful methods to investigate chemical structures in an unperturbed state and therefore free of intermolecular forces for example in liquids or crystals. X-ray diffraction (XRD) is a routine technique in chemistry nowadays, yet the basic principles of gas-phase electron-diffraction (GED) are the same. The comparison of structures in different phases tells us about intermolecular forces and how molecules become distorted in the solid state. Gas-phase structures are unperturbed by such forces and can therefore be compared with quantum-chemical results of free molecules and thereby serve as reference for development of new theoretical methods.
In this project, we investigate and seek to understand intramolecular interactions which influence the structure, reactivity and stability of different molecules in the context of London dispersion forces, halogen bonding, intramolecular stacking interactions and closed shell metallophilic interactions. An essential part of our work regarding evaluation of gas-phase electron diffraction data utilizes state-of-the-art quantum-chemical resources (in terms of Hardware and Software) which are provided by the PC2 HPC-Systems.
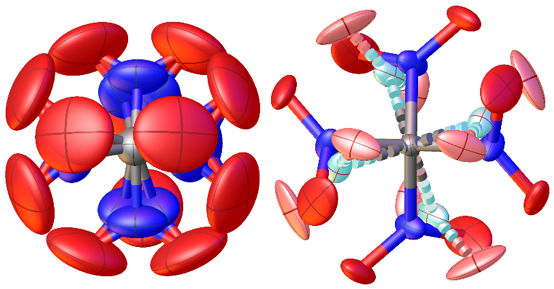
A recent result which illustrates our work is the structural chemistry of tetranitromethane, which was repeatedly investigated for more than 70 years but we could only now describe the dynamics structure in the gas and the complicated disorder in the crystalline state [1].
References
[1] Yu. Vishnevskiy, D. S. Tikhonov, J. Schwabedissen, H. - G. Stammler, R. Moll, B. Krumm, T. M. Klapoetke, N. W. Mitzel
"Tetranitromethane: A Nightmare of Molecular Flexibility in the Gaseous and Solid States"
Angew. Chem. Int. Ed. 2017, 56, 9619-9623