Water is an essential substance for life. Above all, its large heat capacity makes oceans and seas huge heat stores for regulating the Earth's climate. In living organisms, the same property makes water an efficient heat buffer for the functioning of biochemical reactions. So-called hydrogen bridge bonds play a central role in this process. However, the energy flow in this complex network of bonds has not yet been fully investigated at the molecular level. Scientists at the Paderborn University have now succeeded not only in measuring these bonds, but also in influencing them in a targeted manner using a high-energy laser. Their results have now been published in the renowned scientific journal "Science Advances".
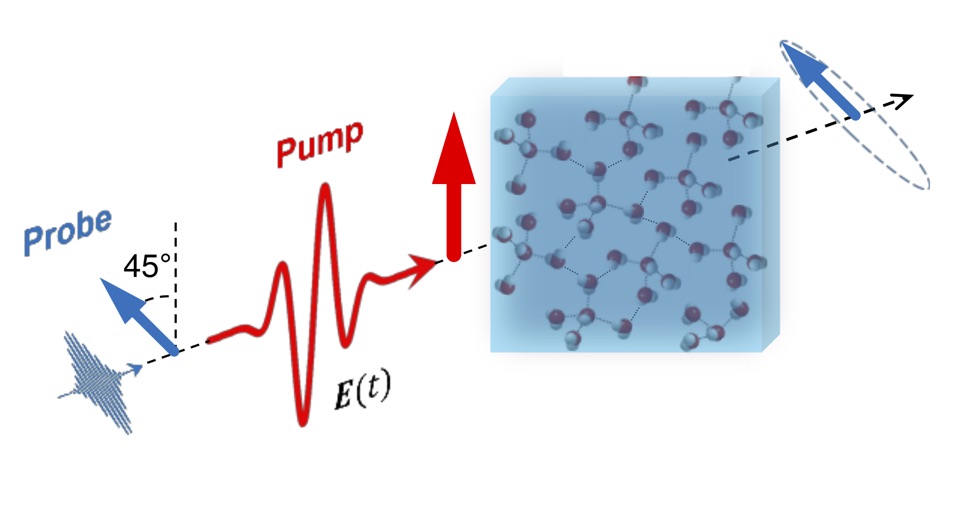
"The primary question is how the energy induced by a terahertz laser is distributed in hydrogen-bridge-bound liquids, how this can be controlled and thus specifically used in chemical reactions. A simple example of such processes is water heated in a microwave oven," says Prof. Dr. Thomas Kühne, Professor at the Department of Chemistry and vice chairman of the PC² management board, who conducted the study together with his colleague Dr. Hossam Elgabarty.
The scientists used a terahertz laser to influence the complex structure of the network, thereby determining the structural dynamics of water. Elgabarty says: "In order to elucidate the molecular mechanism of energy dissipation, i.e. the conversion into heat, and to understand the role of the collective intermolecular movements in this process, we determined the time scale of energy dissipation and also the strength of the intermolecular interactions by means of extensive ab-initio molecular dynamics simulations and energy decomposition analysis using the CP2K suite of programs.“ The latter has been made possible by the recently installed supercomputer Noctua at the Paderborn Center for Parallel Computing (PC²).
The results also enabled the chemists to provide for the first time indications of the asymmetry of the hydrogen bond network, which had previously been predicted theoretically. "With the help of the laser, which can probably specifically cause this asymmetry, it would then be possible, for example, to catalyze "on-water" reactions, which are the subject of our "GreenOnWaterCat" ERC project," says Kühne. These are catalytic processes that take place on water surfaces. Water could thus become not only a sustainable but also a particularly attractive solvent for a large number of relevant synthesis processes.
Link to article: https://advances.sciencemag.org/content/6/17/eaay7074.full